3.1 - Introduction#
The concept of a phase transition is something we are all already familiar with, though in general this idea has only been considered for relatively simple systems such as a ‘perfect’ crystal melting into a liquid. We also touched on the idea of a glass being formed when such a simple system does not form but there is still a change in the macroscopic properties at some characteristic temperature.
In this topic we are going to really dig down into the details of what a phase transition is for both simple kinds of matter and then extend into soft matter systems. The two broad categories we consider are
Equilibrium structures that form through a phase transition and are generally stable (and also rather boring - think a solid crystal below the melting temperature).
Non-equilibrium structures are transient in that a change to some external or control parameter (e.g. temperature, composition) the structure may change to a lower free energy configuration.
Phase transitions almost always involve a change in the order of a state, typically from a more ordered to a less ordered state such as the transition from a solid to a liquid. We can understand this in terms of the balance between entropy and energy often in terms of a change in temperature. This balance between the entropy and energy is captured by the free energy of a system which, in the case for constant volume, is described by the Helmholtz free energy
Let us define an order parameter for a phase transition that takes a zero value in the disordered phase and a finite value in the ordered. We can understand the nature of a phase transition if we understand how this order parameter varies with temperature. If this order parameter changes disontinuously (e.g. melting a crystal) then the phase transition is first order whereas a continuous order parameter describes a second order phase transition.
We also need to consider the timescales in which these phase transitions occur. If a liquid is cooled to the melting temperature then the atoms will not spontaneously arrange themselves into a lattice - this takes time. If these timescales are long then some interesting but intermediate structures can be formed, and sometimes the lack of structure that is ‘locked in’ gives rise to a distinct phase that is neither first nor second order, i.e. glasses.
There are many types of these phase transitions in more interesting materials, some of which are shown in Fig. 43, so we are going to limit ourselves to considering two simple but insightful systems: unmixing of two liquids and crystallisation.
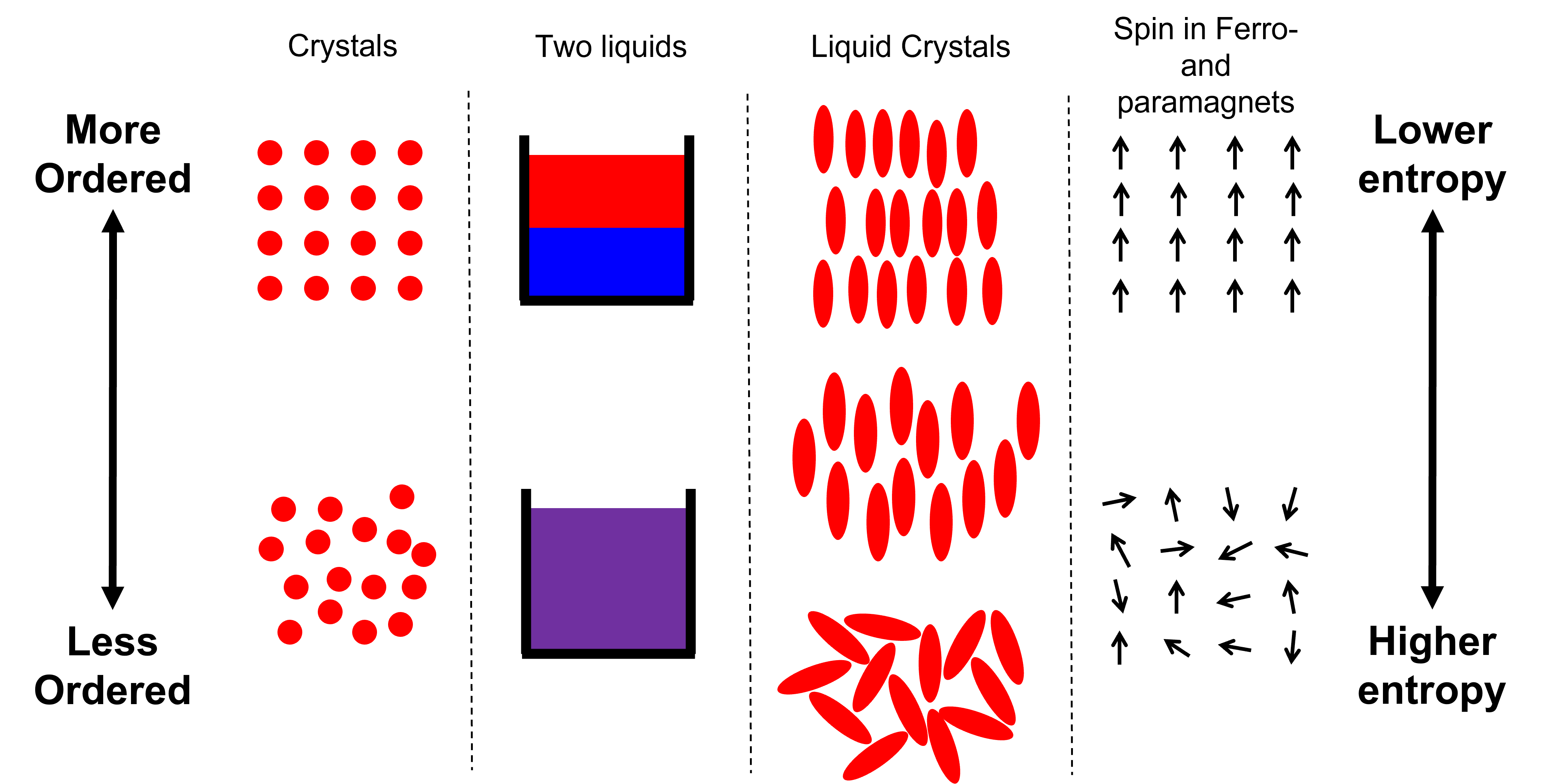
Fig. 43 Some examples of systems in their more and less ordered states.#
